Up to Date Focus on Prevention in Nutrition
Laura Di Renzo and Antonino De Lorenzo
From the University of Rome “Tor Vergata”, Division of Clinical Nutrition and Nutrigenomics, Department of Biomedicine and Prevention, Italy
The chronic non-communicable diseases (CNCDs), including obesity, cardiovascular disease, diabetes, chronic kidney disease, osteoporosis, sarcopenia, Alzheimer's disease, chronic obstructive pulmonary disease (COPD) and cancer, have had a rapid increase in the last decade, spreading globally as an epidemic infectious.According to projections, there will be a further increase of 15% globally between 2010 and 2020, causing 44 million deaths for CNCDs.1 Therefore, it is required a turnaround, providing new preventive strategies2 and targeted interventions for the general and at high diseases risk population.1 To this end, it is necessary to utilize new tools and predictive biomarkers for identifying the risk factors that contribute to the onset of CNCDs, grouping them based on metabolic factors, body composition and genetic profiles.3
One of the common determinants of CNCDs development is undoubtedly food habit, in qualitative and quantitative terms, so as to define them as “food related” pathologies,4 resulting in a significant impact not only on the quality of life, but also on the economy, for the individual, social and health costs.Moreover, it has been established that certain types of food, such as fats and sugar, cause addiction. “Food addiction” has the same symptoms of drug addiction, and is associated with increased impulsivity and emotional reactivity.5 In particular, the person loses the ability to control and limit the intake of food. Neuroimaging studies have further confirmed that the same reward-related regions are activated, for example the medial orbitofrontal cortex. The effects are similar to those seen on addictive and nonaddictive drug consumption, e.g. heroin or aspirin. Foods that cause this behavior are not only unrefined foods, such as vegetables, fruits, and natural fat, but also greatly applies to processed food, e.g. refined carbohydrates or fats, or food that has been subjected to industrial processes, with the addition of substances which increase the palatability of food. These “highly processed” foods are involved in the same biological pathway of drugs of abuse, having the same pharmacokinetic characteristic.5
Evidence that diet is a key environmental factor affecting the incidence of many chronic diseases is overwhelming. The nutritional transition, represented by dietary changes to incorrect habits and physical inactivity, observed especially in high-income developing western countries, but which is now affecting even countries in economic development, is undoubtedly a major modifiable determinant of CNCDs.6 New dietary strategy might significantly help to preserve health status and well-being.
Geographical epidemiologic studies have allowed better understanding of the individuals, their bioanalytic profiles and the possibility of pharmacological and nutripharmacological approaches.
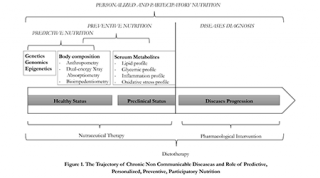
Today we observe new forms of care,7 thanks to the recent development of technologies applied to the field of molecular biology and genetics, which brought medicine from a standby medicine to a predictive and personalized medicine, the so called Predictive, Personalized, Preventive and Participatory (P4) medicine.8 This change is easily applicable to the field nutrition, and can lead to the development of new methods of diagnosis and personalized dietary intervention, on the basis of genetic makeup (nutrigenetic and nutrigenomic assessment), metabolic profile (clinical and biochemical assessment), body composition and energy expenditure (nutritional status assessment). 9-11
The necessity to explore the role and mechanisms of action of nutrients, interpreting the molecular and cellular basis of individual variations and understanding genotype-environmental interactions, thus focusing on relationships between chronic-degenerative diseases and nutritional history, has led to research in human nutrition and medicine on food production optimization for subpopulations with given genetical, ethnical, cultural, and economical settings.12-13
There is a mutual modulation between cellular events and bioactive food components (BFC), that constitutes real effective nutritional homeostasis.3,14 Changes are evident on various molecular levels, involving DNA (i.e., nutrigenetics), pre-transcriptional modifications (i.e., methylations and epigenetics),15,16transcription of mRNA (i.e., nutrigenomics),17 proteins (i.e., proteomics),18 and low molecular weight metabolites (i.e., metabolome and as such metabolomics).19 The use of combined genetic analysis for the study of body composition is an undoubted advantage in the prediction of a risk of disease, which can be reduced or eliminated with simple but targeted personalized nutritional interventions, so as to greatly reduce complications, the cost of drug therapies and hospital interventions.20-22
Environment in which we live may predispose the physiological or pathological responses according to a different genetic predisposition, and environmental factors recombined with genetic ones determines our phenotype.Some individuals, for example, may respond differently to an obesogenic environment, according to genetic differences.23 The current view of adipose tissue is that of an active secretory organ, sending out and responding to signals that modulate appetite, energy expenditure, insulin sensitivity, endocrine and reproductive systems, bone metabolism, inflammation and immunity.24 Nowadays it is necessary to classify the condition of obesity on the basis of body fat composition and distribution, rather than simply on the basis of increased body weight.25 Therefore, BMI, usually used in population studies to correlate overweight and obesity to morbidity and mortality, leads to large errors and misclassifications. Diagnosis, therapy and follow up of all subtypes of obesity must not be based on a single “body weight” parameter, but on body composition parameters and energy expenditure as prerequisites. Direct body fat mass percentage measurement would be a better tool for diagnosing any kind of obesity. However, a predictive evaluation based not only on anthropometric and body composition, but also on biochemical and molecular biology methods may be useful for accurate classification of these subjects. The new term of adiposopathy (‘‘sick fat’’) clearly defines the pathogenic role of adipose tissue. Taking into account the role of adipose tissue, different obesity phenotypes have been described on the basis of body fat composition, fat distribution, and genetics,26-27 based on a genetic predisposition,9,27-30 rather than a simple increase in body weight, and the Body Mass Index (BMI):31 (1) normal weight obese (NWO);32 (2) metabolically obese normal weight;33 (3) metabolicallyhealthy obese;34 (4) metabolically unhealthy obese or “at risk” obese.35
Not only weight and body compositionare determined by a combination of factors (genetic, psychological, social, cultural),36 but the role of the approximately 100 trillion microbes making up the human “microbiome” must be taken into consideration as they enable humans to digest much
more of what we eat than simple digestion allows, which has implications for clinical diagnosis and treatment of many human diseases. Dietary habits constitute a major factor influencing the diversity of the human gut microbiota and recent studies indicate that there is a link between gut microbiota and inflammatory bowel disease, and metabolic syndromes such as obesity and diabetes. Between these, the metabolic activities of the intestinal flora play a decisive role in obesity, because they facilitate the extraction of calories from foods easing the accumulation of substances, such as fatty acids, in adipose tissue, and at the same time providing energy and nutrients to the same microbial growth.37 A possible relationship between the composition of the microbial gut flora and obesity has been demonstrated, focusing with particular attention on the relative proportions of the two main components of the bacterial microbiota: Firmicutes and Bacteroidetes. A prevalence of the first over the latter in the obese subject has been highlighted.38 Recently, it has been shown that a specific bacterial gut microbiota profile linked with increased extraction of calories has been associated with obesity,39 creating a distinct microbiota signature characterized by a decrease of Bacteroidetes and an increase of Lactobacillus, E. coli, Faecalibacterium. Therefore, obesity is characterized by a diverse microbiota, moreover the microbiota itself together with the host genotype and lifestyle could contribute to the development of this metabolic dysfunction.
Gut composition is also affected by resilience to environmental stress, impairing the cortisol awaking response and emotional reactivity in healthy subjects. On the other hand, it has been shown that psychological stress itself leads to dysbiosis, creating a vicious cycle.As a low-grade of inflammatory state is associated with CNCD and with increases in fat mass, the Italian Mediterranean diet (IMD) rich in whole grains, fruit, vegetables, legumes, walnuts, and olive oil may be effective in reducing the prevalence of this syndrome and its associated cardiovascular risk, as well as other chronic degenerative diseases. Mediterranean-like dietary pattern represents a Therapeutic Lifestyle Change (TLC),that can easily be adopted by all population groups and various cultures in the primary and secondary prevention of major chronic diseases. In particular the recommended composition of the IMD is as follows: carbohydrates, 50% to 60%; proteins, 15% to 20% (of which about 50% comprised of vegetable proteins); total fat, less than 30% (saturated fat, less than 10%; and cholesterol consumption, less than 300 mg per day), and 30 g of fiber. No alcoholic beverages are allowed except for 100 ml/day of red wine. Among the indicators that we now have to assess the quality of our lifestyle, the Mediterranean Adequacy Index (MAI) has proved to be extremely useful, as it allows evaluation of the correspondence between actual diet with dietary style indications based on the Mediterranean Diet. Just consider that the progressive increase of CNCD, which has taken place over recent decades in Southern Italy, is associated with a significant reduction in the MAI, from an average value of 9 in the 60's up to the modern average value of 2. The turnaround occurred in 1982 and remained broadly constant, corresponding to the historical moment at which a radical change in food consumption occurred. Increasing the MAI implicates greater protection and addition of a valid treatment option in obesity and the diseases related to it, resulting not only in reduced healthcare costs, but also in improvement ofthe state of personal well-being and social conditions.
In conclusion, new strategies to improve well-being should be planned considering phenotype, metabolism, and the microbiota together, with the aim of identifying in advance the possibility of onset of the disease in vulnerable individuals that would therefore benefit from a variety of more personalized dietary recommendations.
References
1. WHO. World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization 2011.
2. Lenoir-Wijnkoop I, Jones PJ, Uauy R, Segal L, Milner J. Nutrition economics - food as an ally of public health. Br J Nutr. 2013;109(5):777-84.
3. Milner JA. Incorporating basic nutrition science into health interventions for cancer prevention. J Nutr. 2003;133(11 Suppl 1):3820s-6s.
4. Kant AK. Dietary patterns: biomarkers and chronic disease risk. Appl Physiol Nutr Metab. 2010;35(2):199-206.
5. Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One. 2015;10(2):e0117959.
6. Lenoir-Wijnkoop I, Dapoigny M, Dubois D, van Ganse E, Gutierrez-Ibarluzea I, Hutton J, et al. Nutrition economics - characterising the economic and health impact of nutrition. Br J Nutr. 2011;105(1):157-66.
7. Snyderman R, Williams RS. Prospective medicine: the next health care transformation. Academic medicine: journal of the Association of American Medical Colleges. 2003;78(11):1079-84.
8. Galas DJH, L. Systems Biology and Emerging Technologies Will Catalyze the Transition from Reactive Medicine to Predictive, Personalized, Preventive and Participatory (P4) Medicine. . IBC. 2009;1:1-5.
9. Di Renzo L, Bertoli A, Bigioni M, Del Gobbo V, Premrov MG, Calabrese V, et al. Body composition and -174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Current pharmaceutical design. 2008;14(26):2699-706.
10. Di Renzo L, Bianchi A, Saraceno R, Calabrese V, Cornelius C, Iacopino L, et al. -174G/C IL-6 gene promoter polymorphism predicts therapeutic response to TNF-alpha blockers. Pharmacogenetics and genomics. 2012;22(2):134-42.
11. Pitayatienanan P, Butchon R, Yothasamut J, Aekplakorn W, Teerawattananon Y, Suksomboon N, et al. Economic costs of obesity in Thailand: a retrospective cost-of-illness study. BMC health services research. 2014;14:146.
12. German JB, Roberts MA, Watkins SM. Genomics and metabolomics as markers for the interaction of diet and health: lessons from lipids. J Nutr. 2003;133(6 Suppl 1):2078s-83s.
13. Young VR. 2001 W.O. Atwater Memorial Lecture and the 2001 ASNS President's Lecture: Human nutrient requirements: the challenge of the post-genome era. J Nutr. 2002;132(4):621-9.
14. Muller M, Kersten S. Nutrigenomics: goals and strategies. Nature reviews Genetics. 2003;4(4):315-22.
15. Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science (New York, NY). 1999;286(5439):481-6.
16. Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science (New York, NY). 2001;293(5532):1068-70.
17. Green MR, van der Ouderaa F. Nutrigenetics: where next for the foods industry? The pharmacogenomics journal. 2003;3(4):191-3.
18. Ross J. Control of messenger RNA stability in higher eukaryotes. Trends in genetics : TIG. 1996;12(5):171-5.
19. Weckwerth W. Metabolomics: an integral technique in systems biology. Bioanalysis. 2010;2(4):829-36.
20. Tremblay A, Perusse L, Bouchard C. Energy balance and body-weight stability: impact of gene-environment interactions. Br J Nutr. 2004;92 Suppl 1:S63-6.
21. Fantuzzi G. Adipose tissue, adipokines, and inflammation. The Journal of allergy and clinical immunology. 2005;115(5):911-9; quiz 20.
22. De Lorenzo A, Deurenberg P, Pietrantuono M, Di Daniele N, Cervelli V, Andreoli A. How fat is obese? Acta diabetologica. 2003;40 Suppl 1:S254-7.
23. Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, et al. Association between -308 G/A TNF-alpha polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Disease markers. 2013;35(6):615-23.
24. Di Renzo L, Gratteri S, Sarlo F, Cabibbo A, Colica C, De Lorenzo A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Disease markers. 2014;2014:743634.
25. Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, et al. Oxidative stress in normal-weight obese syndrome. Obesity (Silver Spring, Md). 2010;18(11):2125-30.
26. Di Renzo L, Bigioni M, Del Gobbo V, Premrov MG, Barbini U, Di Lorenzo N, et al. Interleukin-1 (IL-1) receptor antagonist gene polymorphism in normal weight obese syndrome: relationship to body composition and IL-1 alpha and beta plasma levels. Pharmacological research. 2007;55(2):131-8.
27. Di Renzo L, Bigioni M, Bottini FG, Del Gobbo V, Premrov MG, Cianci R, et al. Normal Weight Obese syndrome: role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C-->T genes in the relationship between body composition and resting metabolic rate. European review for medical and pharmacological sciences. 2006;10(5):235-45.
28. De Lorenzo A, Soldati L, Sarlo F, Calvani M, Di Lorenzo N, Di Renzo L. New obesity classification criteria as a tool for bariatric surgery indication. World journal of gastroenterology. 2016;22(2):681-703.
29. Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Progress in cardiovascular diseases. 2014;56(4):426-33.
30. Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? The Journal of clinical endocrinology and metabolism. 2004;89(6):2569-75.
31. Seo MH, Rhee EJ. Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinology and metabolism (Seoul, Korea). 2014;29(4):427-34.
32. O'Connell J, Lynch L, Cawood TJ, Kwasnik A, Nolan N, Geoghegan J, et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS One. 2010;5(4):e9997.
33. Di Renzo L, Tyndall E, Gualtieri P, Carboni C, Valente R, Ciani AS, et al. Association of body composition and eating behavior in the normal weight obese syndrome. Eating and weight disorders : EWD. 2016;21(1):99-106.
34. Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. The American journal of clinical nutrition. 2011;94(1):58-65.
35. Angelakis E, Armougom F, Million M, Raoult D. The relationship between gut microbiota and weight gain in humans. Future microbiology. 2012;7(1):91-109.
36. Drissi F, Raoult D, Merhej V. Metabolic role of lactobacilli in weight modification in humans and animals. Microbial pathogenesis. 2016.
37. Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, Burnet PW. Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology. 2015;232(10):1793-801.
38. Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and immunity. 2010;78(4):1509-19.
39. Fidanza F, Alberti A, Lanti M, Menotti A. Mediterranean Adequacy Index: correlation with 25-year mortality from coronary heart disease in the Seven Countries Study. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2004;14(5):254-8.